Lateral Flow Assay Principle
What is a lateral flow test?
Lateral flow assay (LFA) or lateral flow tests are part of the Point of Care Testing (POCT) for rapid diagnostic, offering quick, user-friendly, and cost-effective solutions for the detection and quantification of a target analyte in samples. They are rapid tests primarily intended for the clinical analysis of biological samples such as plasma, urine, and saliva, often seen in home pregnancy tests or rapid COVID-19 tests.
Lateral flow assay based on Bright-Dtech™ not only allows qualitative detection, as our technology also provides a quantitative result. Our lateral flow assay is the first field test with laboratory performance and without the need for complex laboratory equipment. Rapid tests like lateral flow assays allow the early diagnosis of many diseases and infections to guide the choice of the treatment. The application of these rapid tests with Bright-Dtech™ also extends to areas such as veterinary medicine, agriculture, food, and environmental safety.

The Bright-Dtech™ lateral flow assays are based on the migration of detection bioreceptors conjugated to fluorescent nanoparticles along the strip for analytes detection. The resulting signal intensity at the control and test lines is measured by a time-resolved fluorescence reader.
Lateral Flow vs PCR: Which difference?
While both lateral flow and PCR (Polymerase Chain Reaction) tests are pivotal in detecting pathogens, including viruses, they operate on fundamentally different principles. Lateral flow assays offer a 20-minute result with no need for specialized equipment, making them ideal for point-of-care testing and field use. In terms of cost, they are 50 times cheaper than PCR tests. PCR tests, on the other hand, require laboratory conditions and more processing time, leading to long delays in obtaining results. Poly-Dtech combines the advantages of these two techniques, offering lateral flow tests with the same performance as PCR tests.
Lateral Flow protocol
In the Bright-Dtech™ lateral flow assay protocol, the key steps are: preparation of samples, a 20-minute migration of the sample on the test strip, and measurement using a time-resolved fluorescence reader. Our customized Bright-Dtech™ technology is available for all matched antibody pairs developed for classical sandwich ELISA allowing accurate detection and high signal/noise ratio for reliable results.
Sandwich vs. Competitive Lateral Flow Assays
Lateral flow assays (LFAs) are widely used for rapid and reliable biomarker detection. The two main formats — sandwich and competitive — offer distinct advantages depending on the target and application.
- Sandwich LFA is ideal for detecting large biomarkers with multiple binding sites. It uses two antibodies: one immobilized on the membrane and another labeled with Bright-Dtech™ technology, ensuring high sensitivity and specificity. This format provides a strong signal, even for low-concentration targets.
- Competitive LFA is used when detecting small molecules or analytes with a single binding site. In this format, the target competes with a Bright-Dtech™-labeled tracer for binding to an immobilized antibody. The signal intensity is inversely proportional to the target concentration, making it ideal for hormone, toxin, or drug detection.
With Bright-Dtech™, both formats deliver superior performance, providing high sensitivity, fast results, and robust detection across various applications.
Bright-Dtech™ works not only with antibodies but also with other proteins, oligonucleotides, and nucleic acids such as DNA and RNA, enabling versatile applications across different assay formats.
Lateral Flow benefits with Bright-Dtech™ ?
Bright-Dtech™ maximizes lateral flow assay performance providing the same sensitivity of an ELISA assay, with a 20-minute result without the need for complex laboratory equipment. Our technology based on lanthanide nanoparticles offers exceptional sensitivity and specificity, low background noise and user-friendly protocols. This makes our lateral flow test the first field test with laboratory performance, eliminating false positives.
Example: LFA for PSA detection in sandwich format
Prostate-specific antigen (PSA) is a crucial biomarker used in the screening, diagnosis, and monitoring of prostate-related conditions, including prostate cancer. Elevated PSA levels in blood can indicate prostate abnormalities, making it an essential tool for early detection and disease management.
Our Bright-Dtech™ PSA Lateral Flow Assay offers a rapid, sensitive, and reliable solution for PSA quantification, providing clinicians and researchers with an easy-to-use method for real-time assessment of prostate health.
Figure 1. Strips and standard curve used to determine standard curve for PSA detection
This figure demonstrates a very low limit of detection of PSA through TRF reader, which is the same range of sensitivity as PSA ELISA tests.
The system achieved a limit of detection (LoD) of 15 picograms/mL of PSA, which significantly outperformed the visual LoD and demonstrated sensitivity comparable to that of commercial ELISA tests.
This sensitivity, along with the reliability of the reading mode, enabled the successful quantification of patient serum samples. The results obtained are comparable to those achieved with the reference method (Siemens) used by medical analysis laboratories (Figure 2) , further demonstrating the effectiveness of Bright-Dtech™ lateral flow tests.

Figure 2. Comparison of PSA quantification using the reference method and the Bright-Dtech™ LFA
Further evidence of the effectiveness of our PSA Bright-Dtech™ LFA can be found in the following study: ref (coming soon)
Example: LFA for LDH detection in sandwich format
Human Lactate dehydrogenase (h-LDH) is a crucial enzyme involved in cellular metabolism, widely recognized as a biomarker for tissue damage, inflammation, and various pathological conditions. Elevated LDH levels in serum can indicate cell injury, making it a valuable tool for diagnosing and monitoring conditions such as infections, cancer, and cardiovascular diseases.
Our Bright-Dtech™ h-LDH Lateral Flow Assay provides a rapid, reliable, and sensitive solution for LDH quantification, offering researchers and clinicians an easy-to-use method for real-time assessment of cellular health and disease progression.
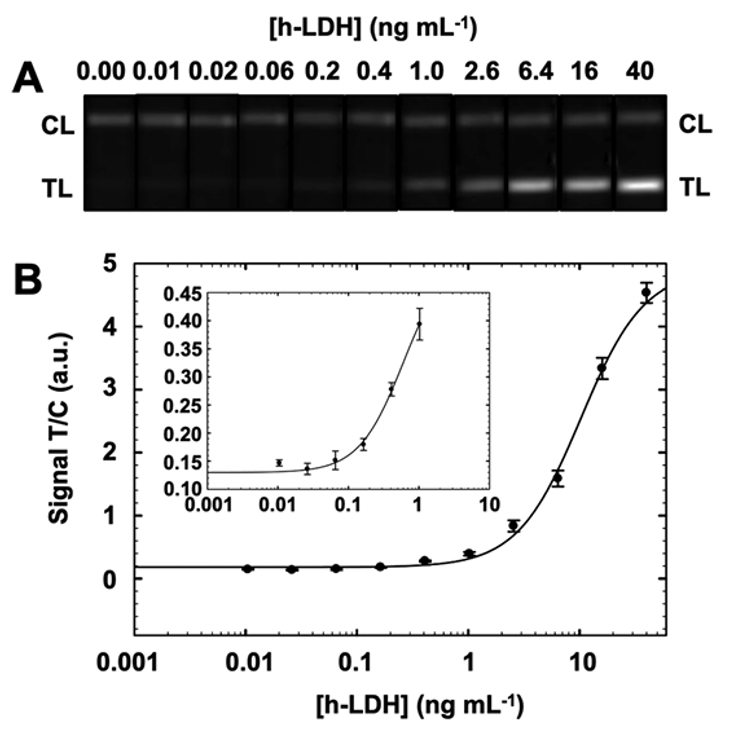
Figure 3. A) Images of the EuNPs-based LFIA strips after detecting serial dilutions of h-LDH in working buffer. (B) Calibration curve plot representing the normalized signal in the TL achieved when detecting serial dilutions of h-LDH standard (Source: J.Lajoux & al.)
The system achieved a limit of detection (LoD) of 38 picograms/mL of LDH, a remarkable sensitivity enhancement by factors of 686, 15, and 2.9 compared to traditional LFIA platforms using AuNPs, CNPs, and even the well-established ELISA, respectively.
Further evidence of the effectiveness of using Bright-Dtech™ nanoparticles to enhance the sensitivity of lateral flow assays can be found in the following study: J. Lajoux et al.: Breaking the picomolar barrier in lateral flow assays using Bright-Dtech™ 614 – Europium nanoparticles for enhanced sensitivity, Microchemical Journal, Volume 209, 2025, 112864. https://doi.org/10.1016/j.microc.2025.112864.


