TR-FRET Assay Principle
What is TR-FRET?
TR-FRET assay is an advanced principle that combines time-resolved fluorescence (TRF) with Fluorescence Resonance Energy Transfer (FRET) to provide sensitive and specific measurements. TR-FRET is ideal for molecular interaction studies, analyte detection, and various applications in biological research. Indeed, it can detect proteins, cytokines, hormones, antibodies, and more present in serum, plasma and cell culture supernatant.
The TR-FRET assay principle is based on the energy transfer between two fluorophores, a donor and an acceptor, when they are in close proximity. By leveraging the delayed fluorescence of the donor and acceptor pair, TR-FRET assay minimizes background signal and increase assay sensitivity, offering a powerful tool for researchers.

In the Bright-DtechTM TR-FRET assay principle, the first antibody is conjugated to our fluorescent nanoparticles (donor) and the second one is coupled with fluorescent molecules (acceptor). If both antibodies recognize the target, the fluorescent intensity from the donor is transferred to the acceptor. Then, a signal can be detected using a time-resolved fluorescence reader to obtain specific and quantitative results.
Required material : Black microplates, Plate shaker, Time-Resolved Fluorescence microplate reader.
TR-FRET vs FRET: Which difference?
While both TR-FRET and FRET are based on the energy transfer between a donor and an acceptor molecule, the key difference lies in the measurement technique.
TR-FRET assay principle incorporates a time-resolved aspect, measuring the fluorescence emission at a delay after the excitation of the donor. This delayed detection allows for the reduction of background noise and increases the signal-to-noise ratio. The delay also enables for a significant stokes shift, which prevents the overlap of emission and excitation peaks, resulting in enhanced sensitivity.
In contrast, traditional FRET measures the immediate energy transfer and may be more susceptible to background fluorescence, affecting sensitivity and specificity.
TR-FRET Protocol
In Bright-DtechTM TR-FRET protocol, the key steps are: preparation of samples, addition of the donor and acceptor, incubation, and measurement using a time-resolved fluorescence reader. TR-FRET assay is a mix-and-read method with a incubation step after the addition of the detection reagents.
TR-FRET Benefits with Bright-DtechTM
Bright-DtechTM maximizes TR-FRET assay performance though high sensitivity and specificity, low background noise and user-friendly protocols. This makes TR-FRET an invaluable technique for drug discovery, molecular diagnostics, and biochemical research.
Example: TR-FRET assay assessing human CRP
C-reactive protein (CRP) is a key biomarker of inflammation, widely used in clinical and research settings to assess immune responses and disease progression. Accurate and sensitive CRP quantification is essential for diagnosing infections, monitoring chronic inflammatory diseases, and evaluating treatment efficacy.
The Now-Dtech™ Human CRP Assay Kit offers a high-performance, wash-free solution for rapid and precise CRP measurement.
For CRP detection using our TR-FRET protocol, samples or standards are added to the microtiter plate wells along with detection reagents. After incubation, Time-Resolved Fluorescence (TRF) is measured at a specific wavelength. The signal intensity is directly proportional to the CRP concentration in the sample, ensuring precise and quantitative detection.
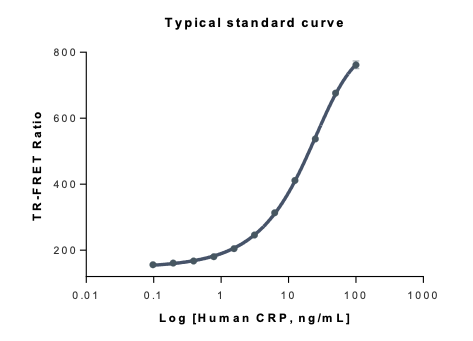
Figure 1. Measurements for human CRP detection though TR-FRET assay principle
Bright-DtechTM technology enhances the detection of human ICRP due to its high specificity and sensitivity given by the time-resolved fluorescence signal in FRET.
Assay range: 0.6-100 pg/mL
LOD: 110 pg/mL

